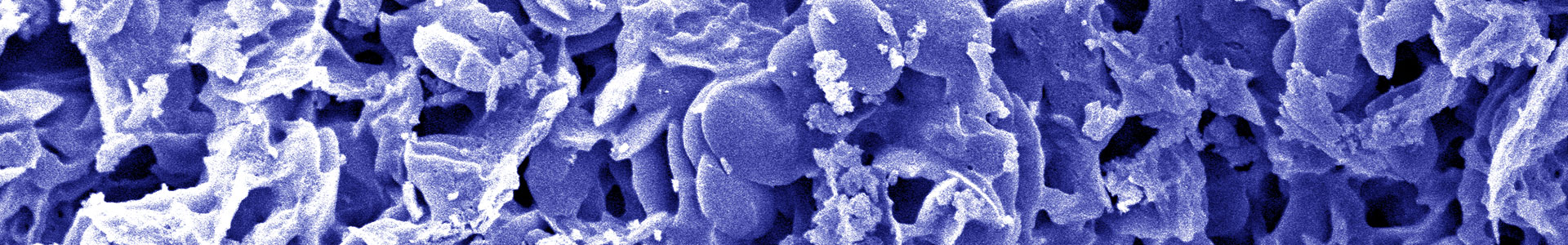
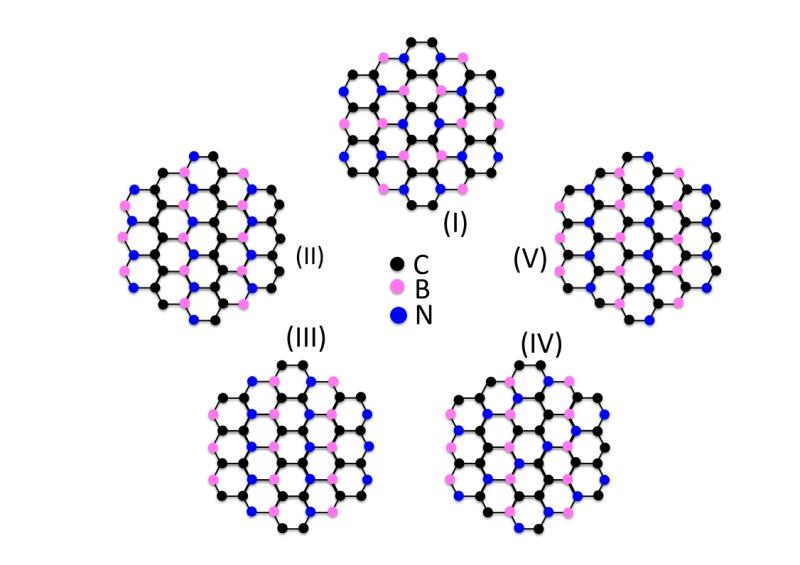
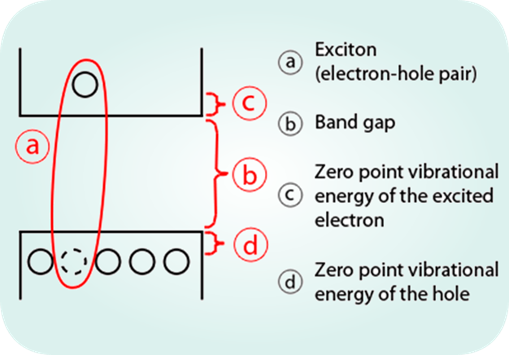
2Dto3D offers boron carbon nitride, a new 2D material, to laboratories and industries to create new devices and improve their products.
Boron carbon nitride is a 2D material with a several potential applications with are a complement to those offered by boron nitride and polymeric carbon nitride, such as:
- Photocatalyst to oxidize contaminants and colorants
- Photocatalyst to produce hydrogen from water
- Transparent photovoltaic cells (for buildings and cars)
- UV-absorber
- Catalyst to specific organic reactions
- Organic semiconductor to optoelectronics
- Fire retardant
- Phosphor to lighting applications and electronic screens
- Fluorescent material